Recall of Valsartan containing drugs
The Inspectorate of Public Health, Social Development and Labor informs the public that there is a recall for a number of blood pressure medications containing the active ingredient valsartan. Valsartan is off-patent and is used as a component of other generic medicines.
This recall has been issued following indications of the presence of an undesirable impurity
N-nitrosodimethylamine (NDMA) in the valsartan active ingredient, which is manufactured by a facility in China. N-nitrosodimethylamine (NDMA) is a chemical substance classified as potentially carcinogenic.
The valsartan active ingredient from the facility in China has been used by various pharmaceutical manufacturers to produce valsartan-containing medications in Europe of which some are available on Sint Maarten.
There is no acute health threat to patients using the affected medication containing valsartan. The chance that a patient actually develops cancer when the contaminated medication is used is small, as a first European analysis shows.
As a precautionary measure, the valsartan containing medications are recalled on a pharmacy level. Pharmacies are therefore instructed to remove the affected medications from their stock and quarantine them.
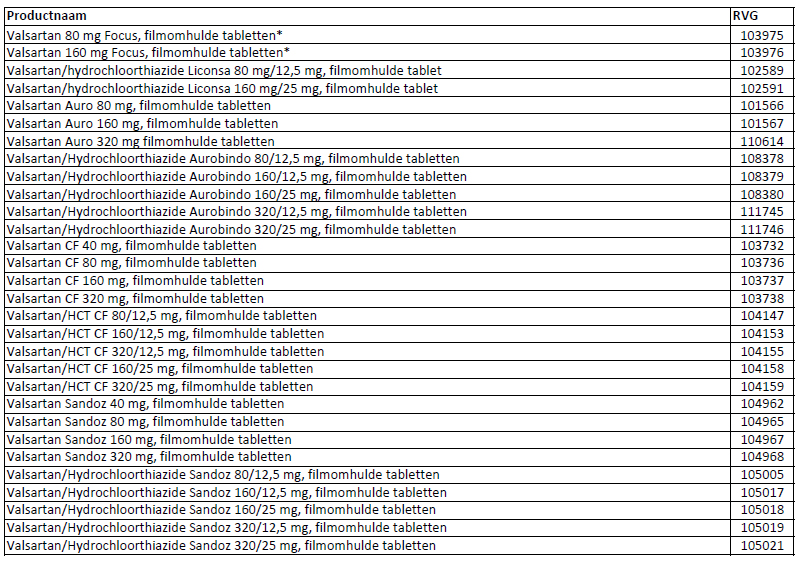
Patients can check whether their medication is affected by this recall by checking the (brand) name and RVG numbers on the medication box using the list added to this press release. The RVG number can be found on the medication box and at the bottom of the leaflet.
It is vital that patients taking valsartan medication affected by this recall do not stop taking their medication abruptly and consult with their doctor or healthcare professional as soon as possible for alternative treatment.